Current Pipeline
At Isosceles, our aim is to provide an opioid alternative with access to as many patients as possible and pledge to be a part of the solution addressing unmet medical needs in postoperative pain space. This policy ensures the safety of our products through rigorous R&D testing and compliance with all FDA guidelines.
IPI201
Synthetic cannabidiol-based Intravenous injection
Acute post operative pain
Isosceles has pioneered a proprietary intravenous formulation based on our synthetic cannabidiol through a patented cGMP-compliant synthetic manufacturing process. IPI201 is a highly bioavailable parenteral formulation that has undergone rigorous analytical testing methods and adheres to the highest quality standards.
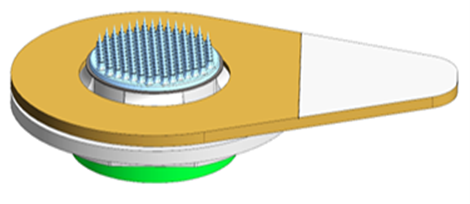
Isosceles is in late-stage development with its exclusive microneedle partner to develop a dissolving microneedle capable of needle-free, intradermal delivery. Our partners have developed a sharps-free platform for vaccines that Isosceles utilizing and modifying in development of a rapid onset, site specific pain relief product.